founded in 2005,leadingpharm (stock code 600222) is a high-quality supplier of pharmaceutical cmc, clinical cro, large and small molecule cdmo and mah and other full-industry chain services to the world.it has been rated as one of the "top ten chinese pharmaceutical r&d companies (ranked no. 1 in 2019/2020)" for many years.
at present,group companies has established cooperative relations with more than 500 upstream and downstream enterprises at home and abroad. we have undertaken more than 600 projects of pharmaceutical r&d and more than 500 clinical research projects, applied for more than 200 patents, and obtained nearly 100 authorizations.
from the beginning of its establishment,group companies took "we make china a world-class player in pharmaceutical technology and production process" as the enterprise vision.and is committed to promoting the development of china's pharmaceutical industry with innovative r&d and service models and information technology!
headquartered in beijing zhongguancun hi-tech park,group companies has a 10000 square meters research and development laboratory, a r&d team with over 1300 people. it can undertake api synthesis, preparation r&d, analysis and testing, project approval, registration, ivivr and other businesses, led by technological innovation to r&d. we establish a high-end api r&d base in miyun, with a laboratory area of 2,000 square meters, which can conduct high-end excipient imitation and substitution research. in zhengzhou linkong biopark investment zhengzhou cdmo base, including macromolecular cdmo platform, small molecule cdmo platform (compliant with fda, ema and nmpa gmp standards) and zhengzhou research institute branch (responsible for solid and liquid dosage formulation r&d). in chongqing investment high-end preparation r&d and mah base, which has a 4,000-square-meter r&d laboratory, responsible for high-end solid preparation and innovative drug, and can provide mah related business. established the first special pediatric research institute in hangzhou, guided by clinical needs, committed to the development of children's preparations and drugs for special groups (e.g. people with dysphagia) by applying for various taste-masking technologies and changing the route of administration.we have a senior r&d management team of more than 1,300 people, a mature drug r&d quality management system and rich project experience, which can provide customers with customized solutions from establishment to commercial transformation with the goal of promoting product launch.

 hot line:010-61006450
hot line:010-61006450
 en
en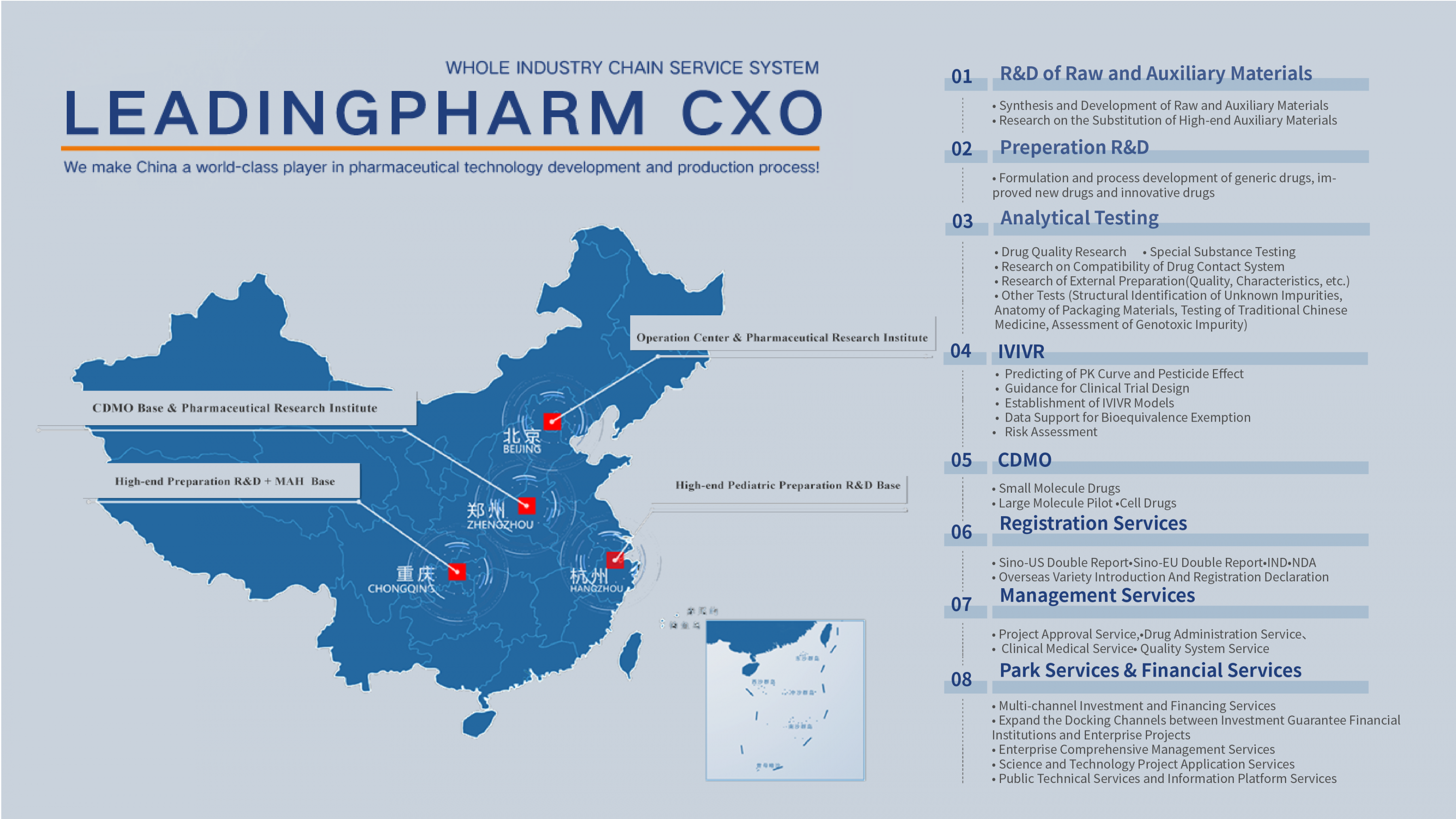

 010-61006450
010-61006450 address:
address: marketing department:
marketing department: leadingpharm
leadingpharm pharm news
pharm news 010-61006450
010-61006450
