good news || congratulate leadingpharm analysis and testing center passed the cnas certification!-九游会电竞
recently, leadingpharm analysis and testing center has obtained the laboratory accreditation certificate awarded by the china national accreditation service for conformity assessment (cnas), which indicates that the company's analytical testing ability and corresponding quality management system have been recognized by international and domestic authorities and can provide customers with more professional and quality services, as well as assurance to product quality control and new drug research.


the china national accreditation service for conformity assessment (cnas) is a national accreditation organization approved and authorized by the certification and accreditation administration of china(cnca) in accordance with the provisions of the regulations of the people's republic of china on accreditation and accreditation. it is responsible for the accreditation of accreditation bodies, laboratories and inspection bodies.
cnas certification marks the leadingpharm analytical and testing laboratory has the technical ability to calibrate / test in accordance with relevant international standards, which will significantly improve the visibility of the laboratory. at the same time, it can also participate in the international laboratory certification of bilateral and multilateral cooperation, be more widely recognized, lay a solid foundation for the building of national key laboratories!
at present, the new leading analysis and testing center has accumulated nearly 100 relevant business experience such as genotoxic impurity detection, polymer impurity research, drug contact system compatibility research and so on. it is believed that with this cnas certification, the new leading will be able to rely on rich project experience, efficient testing methods and outstanding innovation ability to submit high-quality testing services to customers according to cnas approved testing processes and standards, and actively expand the business scope and promote industry development.
thanks to the experts and teachers of cnas for the strict requirements and valuable advice!
introduction of the analytical testing centre
the analysis and testing center of leadingpharm has a professional laboratory of 1,000 square meters, with a highly specialized r & d team and rich experience in testing technology and drug development. it is equipped with more than 40 high-end instruments and equipment in the field of drug analysis, including inductively coupled plasma mass spectrometer (icp-ms), liquid-mass spectrometer (lc-ms/ms), gas-mass spectrometer (gc-ms/ms and gc-ms), ion chromatograph (ic), differential scanning calorimeter (dsc), infrared spectrum (ir), high performance liquid chromatography (hplc, equipped with electrospray detector cad) and gas chromatograph (gc), etc. testing data acquisition and processing systems have network version of the audit tracking function, which can provide safe, accurate, reliable and traceable test data and results for drug r & d enterprises.
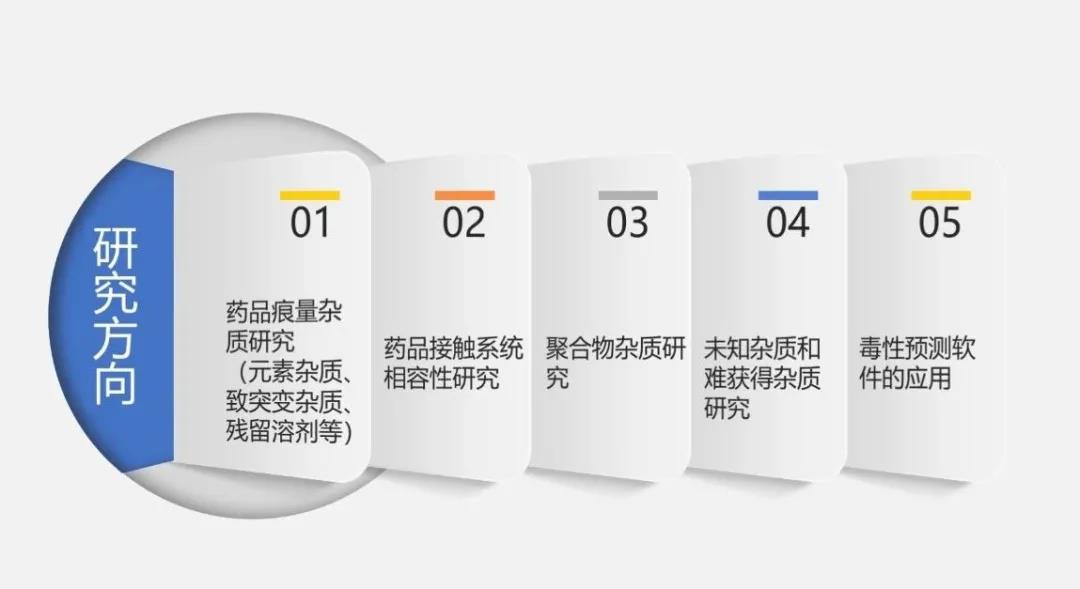
the center's main business areas include: research on the quality of the whole life cycle of drugs in accordance with national drug administration regulations; detection of residual toxic impurities; analysis of trace residues; detection of elemental impurities; study of compatibility of drug contact systems (drug packaging, production components and drug delivery devices, etc.); study of polymer impurities in antibiotics; analysis of unknown impurity structures; study of impurity difficult to obtain mass spectrometry; and use of derek and sarah software for toxicity prediction of impurities containing genotoxic warning structures. among them, polymer impurity research and study of impurity difficult to obtain mass spectrometry are the first technology in the industry, which can provide strong technical support for the difficulties and pain points in the process of drug development.
the center's drug quality research team has nearly 20 years of experience in drug research and development, familiar with national drug laws and regulations and guiding rules, and has participated in hundreds of research projects, including innovative drugs and generic drug formulations and varieties. the team has accumulated rich practical experience, which can meet customers’ research needs of api and preparation quality.
brief introduction of analysis and testing center
the analysis and testing center of beijing new leading pharmaceutical technology development co., ltd. has a professional laboratory of 1,000 square meters, equipped with more than 40 high-end instruments and equipment in the field of pharmaceutical analysis, mainly including inductively coupled plasma mass spectrometer (icp-ms), liquid-mass spectrometer (lc-ms/ms), gas-mass spectrometer (gc-ms/ms and gc-ms), ion chromatograph (ic) and differential scanning calorimeter (dsc)the center has not only passed iso9001 quality management system certification, but also will soon be accredited by cnas.the detection data collection and processing system has the online audit tracking function, which can provide customers with safe, accurate, reliable and traceable detection data and results.
the center not only has highly specialized r&d technical team and rich experience in detection technology and drug research and development, but also has a very strong advisory team composed of well-known experts in the industry , as well as a joint laboratory established with shimadzu and thermo fisher scientific instruments, which can use the most advanced instruments and equipment in the world to provide professional, accurate and fast detection technology services for drug research and development enterprises.
the main business scope of the center includes: research on the quality of the whole life cycle of drugs in compliance with national pharmaceutical regulations , genotoxic impurity detection, trace residue analysis, elemental impurity detection, drug contact system compatibility research (packaging materials, production components and drug delivery devices, etc.), antibiotic drug polymer impurity research, unknown impurity structure analysis, difficult to obtain impurity mass spectrometry research, and using derek and sarah software to predict the toxicity of impurities with genotoxicity warning structure.among them, the research of polymer impurities and mass spectrometry detection of hard-to-obtain impurities are the first technologies in the industry, which can provide strong technical support for the difficult and painful problems in the process of drug research and development.
the drug quality research team of the center has nearly 20 years' experience in drug research and development, is familiar with various national drug administration regulations and guiding rules, and has participated in hundreds of research projects, including various dosage forms and varieties of innovative drugs and generic drugs, and has accumulated rich practical experience, which can fully meet the research needs of customers for the quality of apis and preparations.
environmental equipment display



▲icp-ms ▲icp-ms


▲gc ▲gc-ms


▲lc-ms ▲lc-ms


▲hplc ▲ic


▲elsd ▲微波消解仪


▲ir ▲dsc
research field
study on the quality of medicine and medicine
main business:
1. development and methodology of quality control methods for starting materials and key intermediates.
2. trace analysis of impurities in the research of synthesis process.
3. quality research and standard formulation of raw materials and preparations.
4. identification of unknown impurity structure, which is detected and analyzed by high-resolution mass spectrometer.
trace impurities and residual solvent detection
main business:
1. conduct qualitative and quantitative research on trace impurities in starting materials, intermediates, apis and preparations.
2. development of detection methods and methodology for trace impurities (such as genotoxic impurities).
3. method development and methodology of residual solvents and volatile impurities.
4. use ion chromatograph to detect trace anion and cation impurities.
5. detect trace metal impurities by inductively coupled plasma mass spectrometer.
compatibility of drug contact system
with reference to the regulations and guiding principles promulgated by the state drug administration and relevant foreign institutions, the test scheme was designed for the compatibility research of drug contact system, and simulated extraction test, extract research, adsorption and migration research, safety evaluation and other work were carried out.guided by safety assessment and on the premise of improving the efficiency of drug research and development, the center is committed to providing accurate and rapid solutions for compatibility research of drug contact systems and comprehensive and reliable compatibility research data for rapid drug declaration.
main business:
1, according to the decision tree of packaging materials research, design compatibility research scheme for all kinds of drug packaging and use systems, provide experimental data, and write necessary data for drug declaration.
2. study on the extraction of various materials and additives of drug packaging materials, production components and drug delivery devices.
3. according to the collected production information of drug contact system, focus on the possible or extracted toxic substances, further carry out safety and toxicological evaluation, and give the conclusion of compatibility research.
research on polymer impurities
the center has established joint laboratories with shimadzu and thermo fisher scientific, the world's top instrument and equipment companies, and is committed to cooperating in the development of new detection technologies and methods.
polymers are allergens that trigger the immediate allergic reaction of β -lactam antibiotics, and the study of polymers is one of the key points of antibiotic quality control.at present, gel filtration chromatography (molecular exclusion chromatography), ion exchange chromatography, reversed-phase chromatography and capillary electrophoresis are commonly used to detect polymers, among which gel filtration chromatography is the main method.
in recent years, it has been reported that liquid chromatography-mass spectrometry can be used to detect polymers, but only the total amount of impurities in polymers can be detected, and the structure of individual polymers cannot be separated and identified.we have established a joint laboratory with shimadzu mass spectrometry innovation center, and jointly developed multidimensional chromatography/mass spectrometry technology to separate and identify the structure of polymers.to provide technical reference for enterprises to improve process, research and development of dosage forms, and improve quality, and to ensure the safety of medication for patients.at the same time, it can also provide scientific basis for improving pharmacopoeia standards.
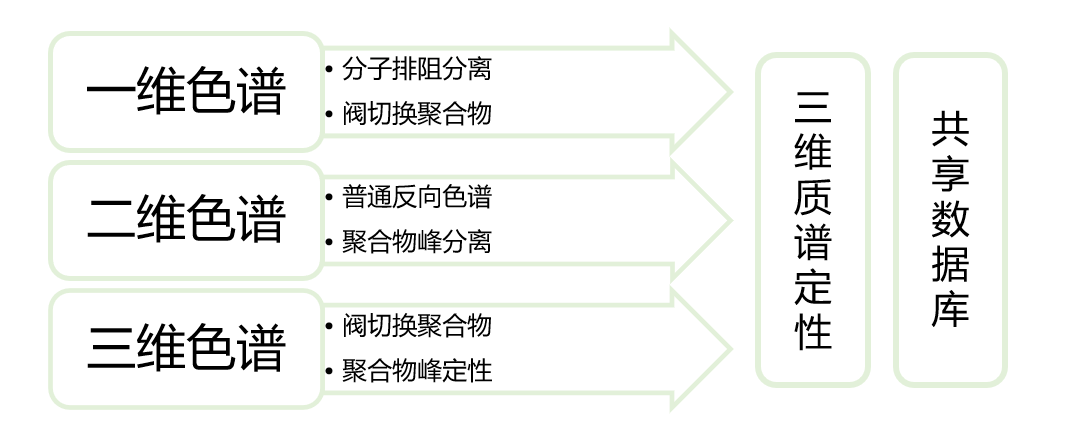
instruments and equipment are shown in the following figure:
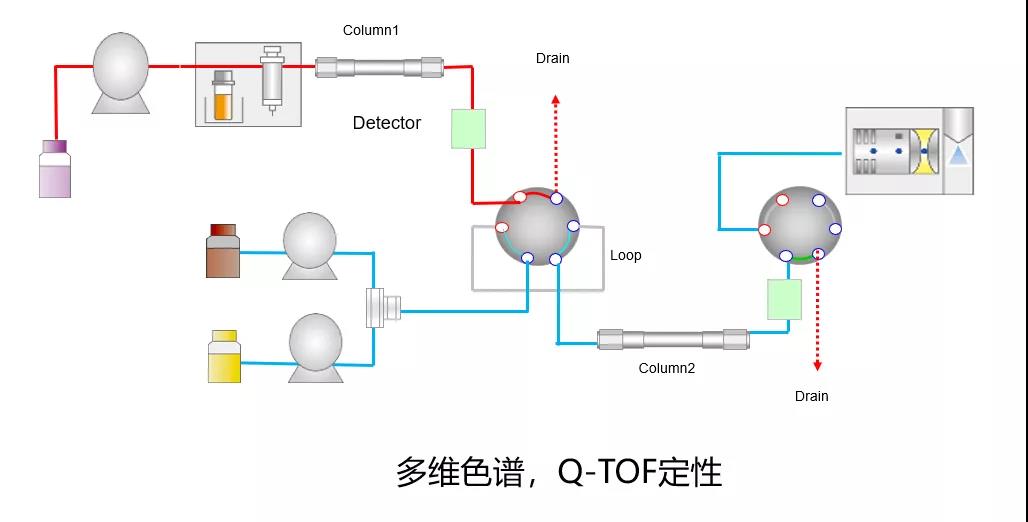
the center and shimadzu joint laboratory have carried out research work on more than 10 polymer impurities of antibiotics, and will continue their cooperation in this field in the future.at present, the database of polymer impurities is being established, and it is expected to share data and achievements with peers in the future.
research on unknown impurities and difficult-to-obtain impurities
the center uses the top-notch high-resolution mass spectrometry instruments and equipment of thermo fisher scientific company, and carries out cooperative research work with it to jointly solve new hot and difficult problems in the field of drug research and development, especially in the research of unknown impurities and hard-to-obtain impurities by using high-resolution mass spectrometry technology, which opens up new ideas and new fields for the quality research of innovative drugs and generic drugs.
in the process of developing pharmaceutical process routes, some processes or degradation impurities can not be purchased and synthesized through commercial channels, and it is very difficult.in this case, on the basis of consulting relevant experts, we plan to establish a lc/ms method to study it.this method can greatly shorten the research period of drug quality, improve the research efficiency, and greatly save manpower, material resources and financial resources.
application of toxicity prediction software
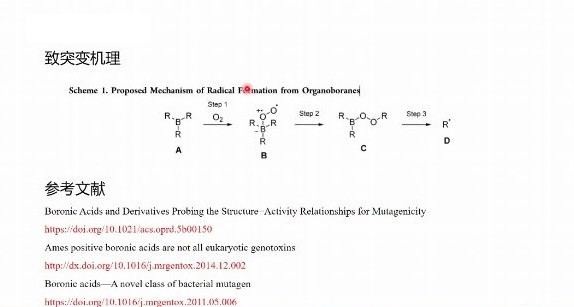
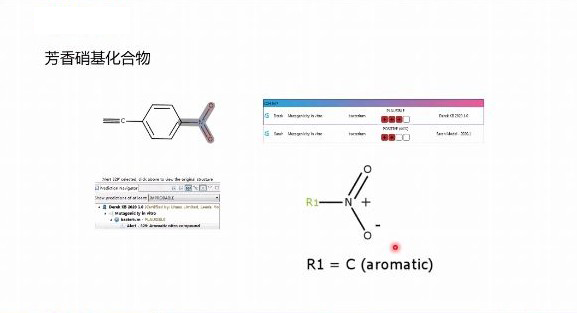
when designing the top-level project, it is necessary to predict its impurities according to the preparation technology and stability of drugs, especially the genotoxic impurities which have attracted much attention in recent years.derek and sarah software are approved by the regulators of ich m7 guiding principles, and developed by lhasa limited in the uk.the software meets the requirements of ich m7 guiding principle for the mutagenicity risk control of impurities, and can meet the requirements of the guiding principle that computational toxicology assessment method should be used instead of bacterial mutagenicity test for impurities, and automatically provide impurity grade classification.at the same time, the prediction results of this software are recognized by cde.the use of this software can greatly improve our research and development efficiency, so that we can provide customers with more efficient and accurate services.
the analysis and testing center of the new leading company is still strengthening the construction of talent team, introducing more talents, expanding the scale of laboratories, using more advanced instruments and equipment, and expanding the scope of testing services.at the same time, we will unite more high-end partners to build a detection technology team with exquisite technology and excellent equipment, jointly explore new detection technology fields, help china's new drug and imitation research and development level to be in line with international standards, and let more people eat cheap drugs and conscience drugs.
-end-


转载声明:未经本网或本网权利人授权,不得转载、摘编或利用其他方式使用上述作品。已经本网或本网权利人授权使用作品的,应在授权范围内使用,并注明“来源:新领先医药科技”。

 hot line:010-61006450
hot line:010-61006450
 en
en

 010-61006450
010-61006450 address:
address: marketing department:
marketing department: leadingpharm
leadingpharm pharm news
pharm news 010-61006450
010-61006450
